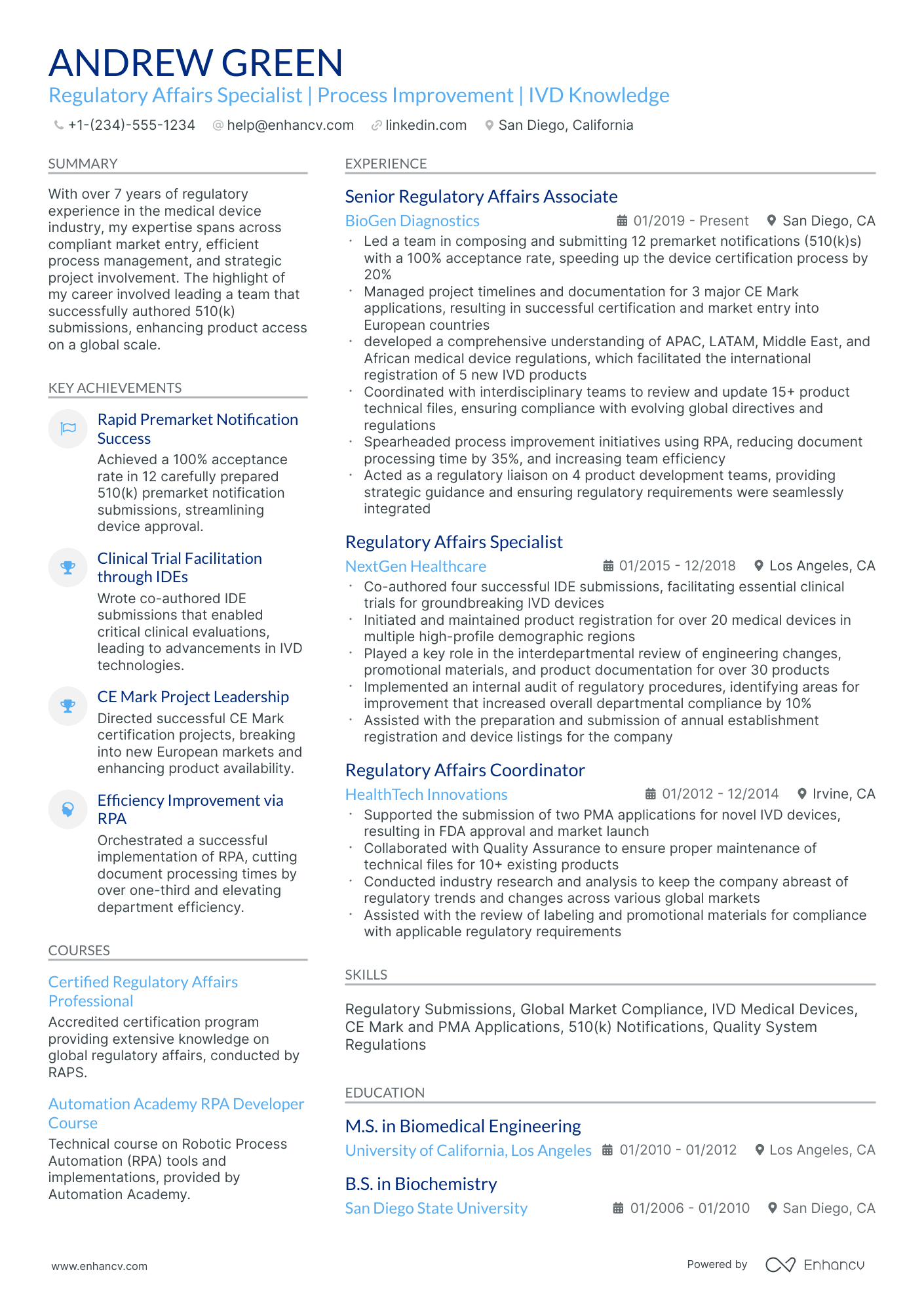
Crafting a resume for regulatory affairs positions can be daunting due to the need to balance technical expertise with an understanding of ever-changing compliance protocols. Our guide offers clear strategies and examples to help you articulate your qualifications and stay ahead in the competitive landscape of regulatory affairs.
- Incorporate regulatory affairs job advert keywords into key sections of your resume, such as the summary, header, and experience sections;
- Quantify your experience using achievements, certificates, and more in various regulatory affairs resume sections;
- Apply practical insights from real-life regulatory affairs resume examples to enhance your own profile;
- Choose the most effective regulatory affairs resume format to succeed in any evaluation process.
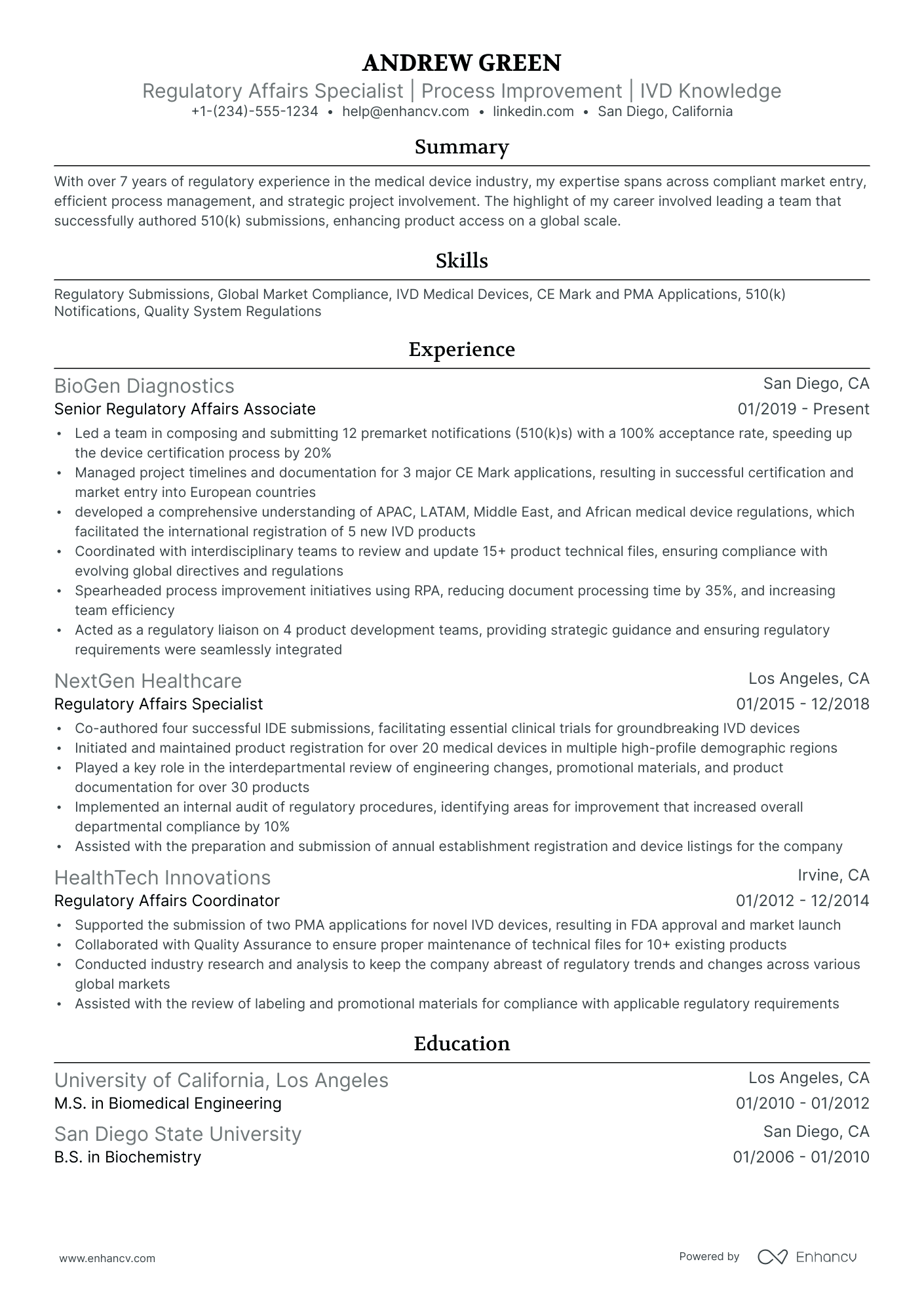
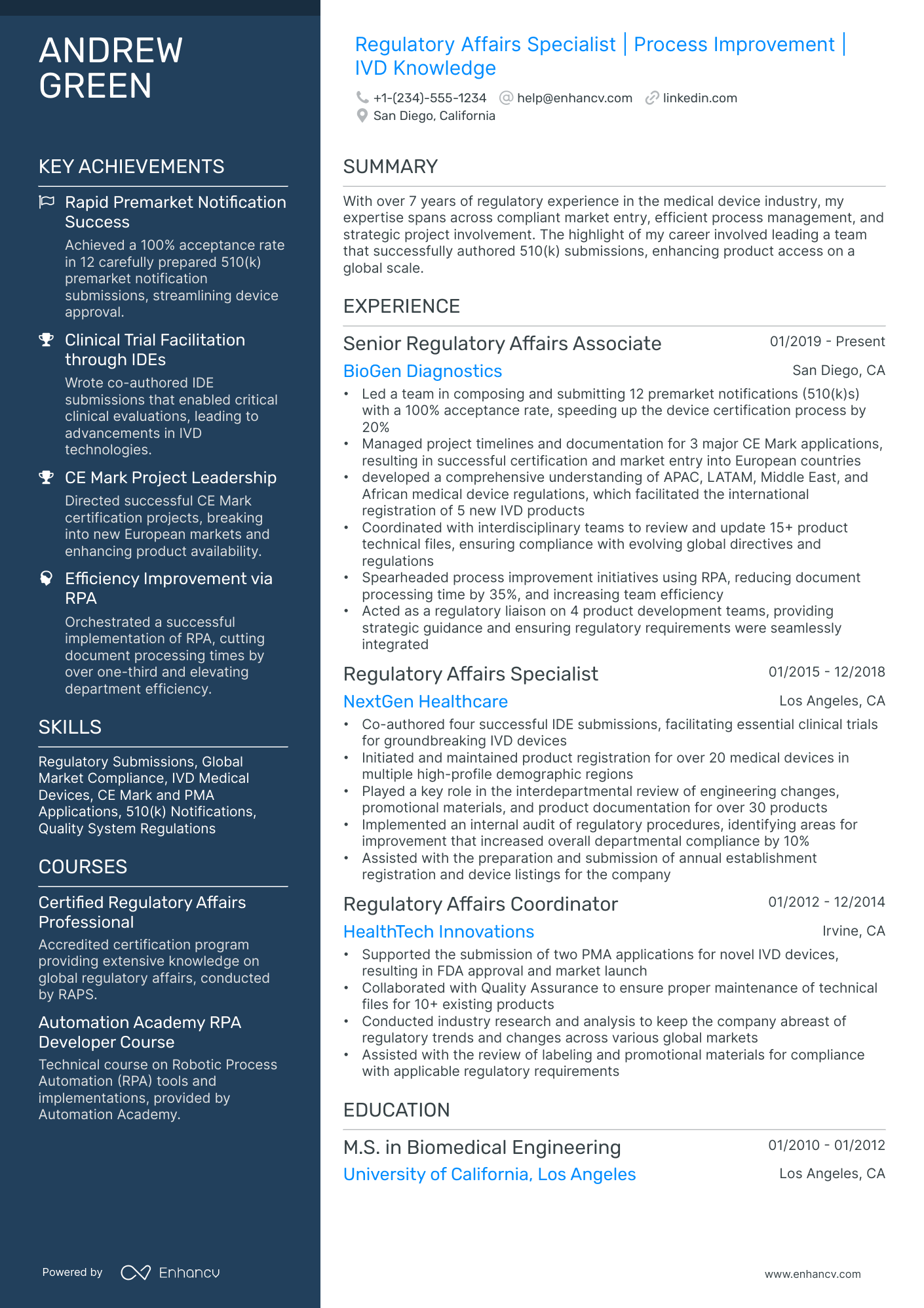
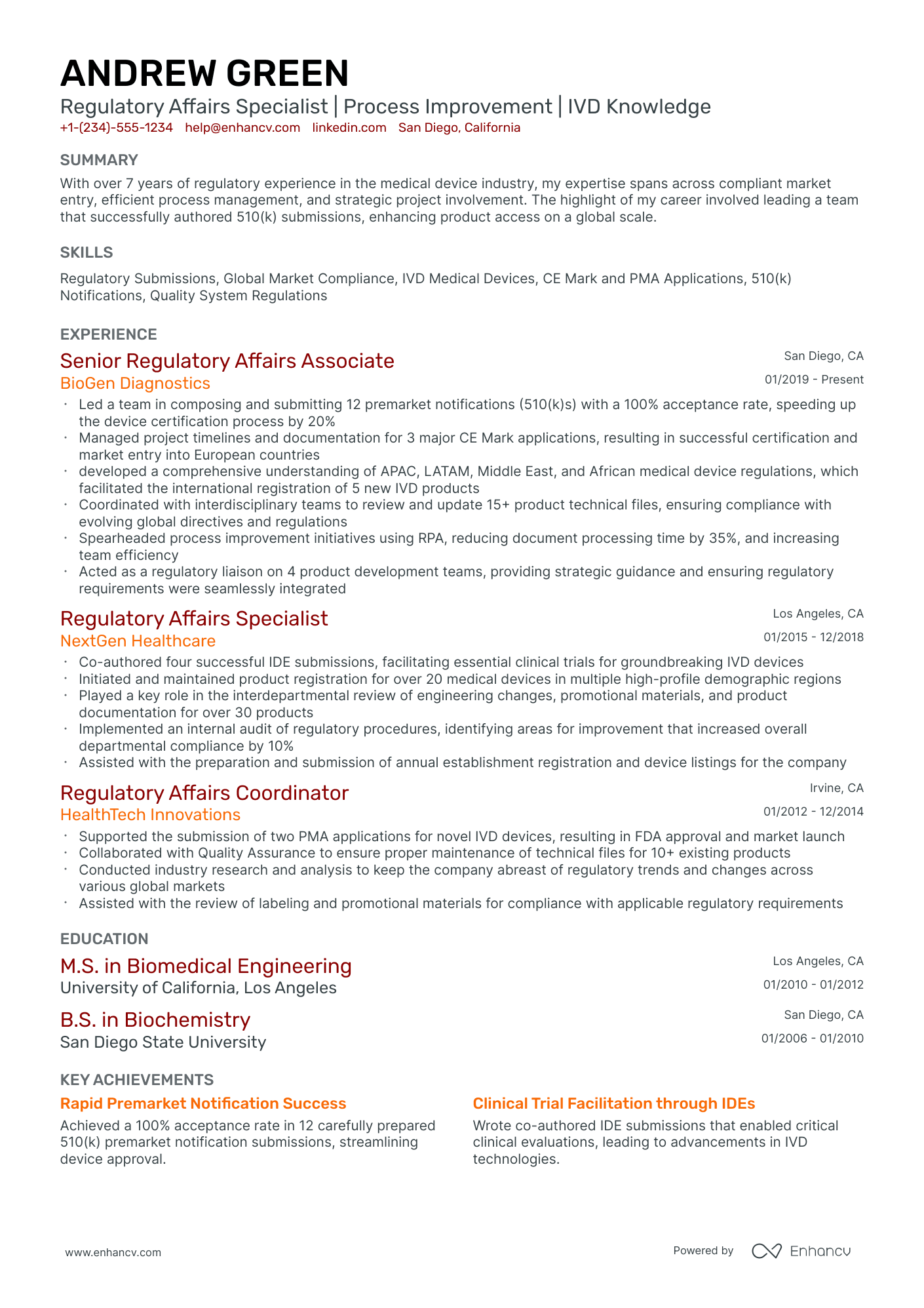
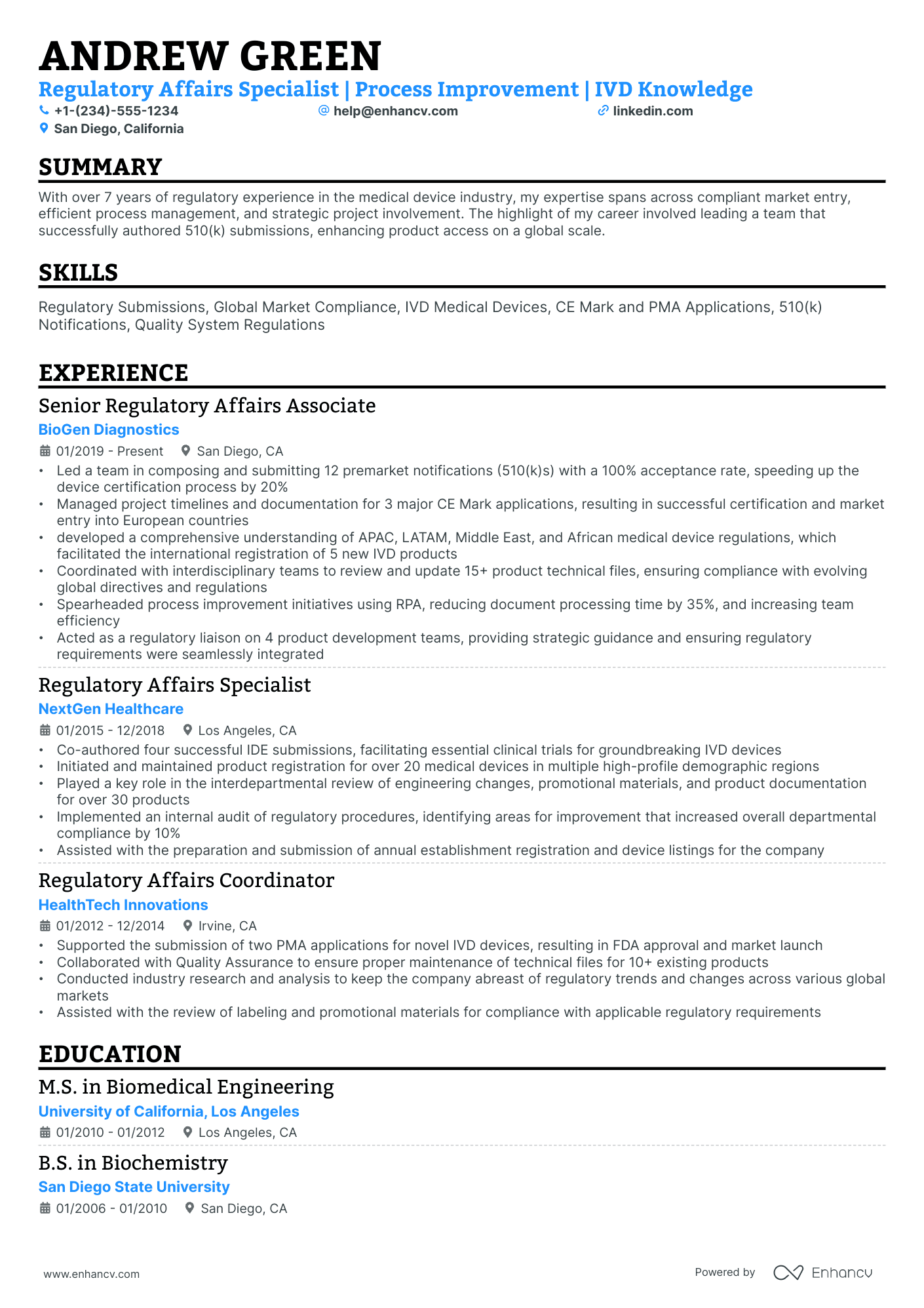
The importance of format and layout in your regulatory affairs resume
Consider you're an HR professional at company X, evaluating two regulatory affairs candidate resumes. John Smith presents a simple, traditional, and easy-to-read resume. Edward Price, however, uses a non-conventional, often illegible format. Whose resume would you spend more time on to understand their experience? This scenario underscores the importance of your regulatory affairs resume’s design. It should be simply formatted and clearly communicate why you are the ideal candidate for the role.
Achieve this balance by:
- Listing your experience, beginning with the most recent and relevant, in reverse chronological order;
- Ensuring your header contains essential information, such as contact details, a headline, and a portfolio link. Include a professional photo in the regulatory affairs resume header if you have one;
- Including only the most important and relevant resume sections to showcase your expertise and stand out from other candidates;
- Editing your regulatory affairs resume to be no longer than two pages if you have extensive relevant experience. Use your limited resume space judiciously.
Also, remember that your regulatory affairs resume might initially be scanned by an Applicant Tracker System (ATS).
When it comes to ATS:
- Opt for simple and legible fonts like Raleway, Rubik, Lato, etc., making your experience easy for the ATS to scan;
- Use serif and sans-serif fonts, both of which are ATS-friendly;
- Avoid overused options like Arial and Times New Roman, which, while suitable, may lack personality.
Contrary to a common myth, our recent study shows that the ATS can effectively process both one-column and two-column resumes. Learn more about this in the ATS myths guide.
Finally, when submitting your regulatory affairs resume, always export it as a PDF to ensure all information remains intact, making the document easier to print, read, and scan.
Customize your resume for the market – a Canadian format, for example, might vary in structure.
Upload & Check Your Resume
Drop your resume here or choose a file. PDF & DOCX only. Max 2MB file size.
PRO TIP
The more time and effort you've put into obtaining the relevant certificate, the closer to the top it should be listed. This is especially important for more senior roles and if the company you're applying for is more forward-facing.
Essential sections that should make up your regulatory affairs resume include:
- The header - with your contact details (e.g. email and telephone number), link to your portfolio, and headline
- The summary (or objective) - to spotlight the peaks of your professional career, so far
- The experience section - with up to six bullets per role to detail specific outcomes
- The skills list - to provide a healthy mix between your personal and professional talents
- The education and certification - showing your most relevant degrees and certificates to the regulatory affairs role
What recruiters want to see on your resume:
- Experience with regulatory submissions (e.g., NDAs, ANDAs, BLAs, MAAs) and knowledge of relevant guidelines (e.g., FDA, EMA, ICH)
- Demonstrated understanding of regulatory strategy and lifecycle management for pharmaceuticals or medical devices
- Familiarity with Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP) regulations
- Strong communication and negotiation skills for interactions with regulatory authorities and cross-functional teams
- Evidence of continued professional development in regulatory affairs, such as RAC certification or related advanced degrees
Writing your regulatory affairs resume experience
Within the body of your regulatory affairs resume is perhaps one of the most important sections - the resume experience one. Here are five quick tips on how to curate your regulatory affairs professional experience:
- Include your expertise that aligns to the job requirements;
- Always ensure that you qualify your achievements by including a skill, what you did, and the results your responsibility led to;
- When writing each experience bullet, ensure you're using active language;
- If you can include a personal skill you've grown, thanks to your experience, this would help you stand out;
- Be specific about your professional experience - it's not enough that you can "communicate", but rather what's your communication track record?
Wondering how other professionals in the industry are presenting their job-winning regulatory affairs resumes? Check out how these regulatory affairs professionals put some of our best practices into action:
- Developed and implemented regulatory strategies for new oncology drugs, leading to successful FDA approvals for three novel therapies.
- Oversaw a cross-functional team that prepared and submitted over 50 INDs and associated amendments, contributing to a streamlined development process.
- Handled regulatory submissions for international markets, achieving a 30% reduction in time-to-market for key products in the European Union.
- Liaised with regulatory agencies to negotiate and define the scope of regulatory submissions, ensuring compliance with local and international standards.
- Led the preparation of an exhaustive Environmental Risk Assessment for a new agricultural biotechnology product, facilitating its clearance.
- Managed complex regulatory projects for medical devices, including the compilation and review of technical documentation, resulting in a 20% increase in approval efficiency.
- Authored comprehensive regulatory dossiers for biologics and was instrumental in obtaining market authorization for two blockbuster immunotherapies.
- Pioneered an initiative to digitize submission processes, greatly enhancing the accessibility and tracking of regulatory documents.
- Played a key role in the cross-departmental task force that addressed compliance issues, significantly reducing potential regulatory infractions.
- Executed due diligence assessments for potential acquisitions, identifying regulatory risks and opportunities that influenced business decisions.
- Contributed to the development of a proprietary regulatory intelligence database, streamlining data retrieval and analysis efforts for the whole department.
- Spearheaded the initiative to train junior regulatory affairs staff, enhancing team knowledge and productivity by 25%.
- Directed regulatory strategy for advanced gene therapy products, playing a pivotal role in the attainment of first-ever FDA approval for a gene therapy treatment in a specific genetic disorder.
- Cultivated productive relationships with key stakeholders at the FDA, EMA, and other health authorities, facilitating smoother communications and negotiations.
- Introduced an automated system for tracking regulatory commitments, which bolstered compliance rates by 35% across multiple product lines.
- Crafted regulatory submission plans that aligned with corporate timelines, optimizing resources and reducing go-to-market time by an average of 4 months.
- Fostered a culture of continuous improvement by instituting regular regulatory process audits, enhancing overall efficiency by 15%.
- Served as the primary regulatory representative in multidisciplinary project teams, ensuring all regulatory requirements were understood and met for each new product launch.
- Managed the submission and maintenance of regulatory filings for a portfolio of over 20 pharmaceutical products, securing prolonged market presence and compliance.
- Strategized and executed a successful pathway for orphan drug designation, leading to tax credits and grant opportunities for the company.
- Orchestrated the creation and delivery of training programs for global regulatory requirements, equipping the team with the knowledge to tackle diverse international markets.
- Engaged actively in the reformulation of clinical development plans in light of changing regulatory guidelines, ensuring no disruptions to ongoing clinical trials.
- Provided expert guidance on the regulatory landscape to influence product development and lifecycle management from a regulatory perspective.
- Conducted rigorous analysis and reporting on post-marketing surveillance data, enhancing patient safety and maintaining compliance with post-marketing commitments.
Quantifying impact on your resume
- Include the number of regulatory submissions managed and their success rate to demonstrate project management and effectiveness.
- List the specific number of countries in which you’ve secured product approvals to show international regulatory expertise.
- Mention the percentage of compliance issues resolved within deadlines to highlight problem-solving skills and efficiency.
- Quantify the amount of training sessions or people you’ve trained on regulatory affairs to establish leadership and knowledge-sharing capabilities.
- Specify the number and type of audits conducted, along with any improvements implemented, to illustrate due diligence and continuous improvement.
- Display the reduction in approval times achieved under your guidance to emphasize process optimization.
- Detail the volume of documentation prepared or reviewed to show thoroughness and attention to detail.
- Report the financial impact of regulatory strategies you’ve implemented, such as cost savings or avoidance of fines.
Action verbs for your regulatory affairs resume
What can candidates do about their resume, if they have no experience
Job requirements can sometimes be answered by other elements you could make more prominent in your regulatory affairs resume.
Thus, you'd be substituting your lack of experience with your relevant:
- Education with details of skills you've obtained that align with the job
- Internships and short-term jobs that are once more dedicated to putting your expertise in the spotlight
- Skills section answering basic and - potentially - more specific job qualifications
- Strengths or accomplishments to show the unique value you present, even as a candidate with less or no professional experience in the industry.
Recommended reads:
PRO TIP
If you happen to have some basic certificates, don't invest too much of your regulatory affairs resume real estate in them. Instead, list them within the skills section or as part of your relevant experience. This way you'd ensure you meet all job requirements while dedicating your certificates to only the most in-demand certification across the industry.
Regulatory affairs resume skills: the essential hard skills and soft skills checklist
Ultimately, your regulatory affairs resume should hint to recruiters that you possess an array of talents that are indispensable to the role.
For example, listing the technologies and software you're apt at using (or your hard skills) and how you apply them in your day-to-day responsibilities would ensure you meet the technical requirements of the role.
But is this enough to ensure that you make a good impression on recruiters?
Go a step further by detailing the soft skills or personality traits you've attained thanks to your work and life experience.
The best way to balance hard skills and soft skills on your regulatory affairs resume is by:
- Highlighting up to three of your most noteworthy career accomplishments in a separate section.
- Listing at least one hard skill and one soft skill you've used to solve a particular challenge or problem.
- Feature niche skills and technologies that would help you stand out amongst candidates.
- Think back on the social impact your efforts have had towards improving the work environment - were you able to always maintain a professional ethic, while enhancing the team culture? Write about your contribution to the role, department, or organization itself as a metric of success.
The skills section of your resume provides you with plenty of opportunities to detail your technical and personal traits.
All you have to do is select the talents that best fit your application and expertise. Make note of some of the most prominent hard and soft skills across the industry from our list:
Top skills for your regulatory affairs resume:
Regulatory Compliance Software
Clinical Trial Management Systems (CTMS)
Electronic Lab Notebooks (ELN)
Document Management Systems (DMS)
Submission Management Tools
Risk Assessment Tools
Data Analysis Software
Regulatory Information Management Systems (RIMS)
Quality Management Systems (QMS)
Labeling and Packaging Software
Attention to Detail
Analytical Thinking
Communication Skills
Problem-Solving
Project Management
Interpersonal Skills
Time Management
Adaptability
Team Collaboration
Negotiation Skills
PRO TIP
Listing your relevant degrees or certificates on your regulatory affairs resume is a win-win situation. Not only does it hint at your technical capabilities in the industry, but an array of soft skills, like perseverance, adaptability, and motivation.
Showcase academic background with education and certifications' sections
Listing your education and certifications should be a rudimentary part of your resume writing.
Including your relevant academic background - in the form of your higher education degree and niche-specific certificates - will prove knowledge of the industry.
For your education section:
- Start by including your degree, followed by start and graduation dates, as well as the institution;
- You could include relevant coursework, major/minor , or GPA, only if your've just graduated from college or if this information would further support your application;
- If you have an "ongoing" degree, you can still list it in case you think your diploma can impress recruiters or it's required;
Follow a similar logic for your certifications section by listing the institution, alongside dates you've obtained the certificate. For some of the most recent and relevant industry certificates , check out the next part of our guide:
The top 5 certifications for your regulatory affairs resume:
- Regulatory affairs Certification (RAC) - regulatory affairs Professionals Society (RAPS)
- Certified in Healthcare Compliance (CHC) - Compliance Certification Board (CCB)
- Certified Regulatory Compliance Manager (CRCM) - American Bankers Association (ABA)
- Medical Device regulatory affairs Certification (MDRAC) - Association of International Certified Professional Accountants
- Pharmaceutical regulatory affairs Certification (PRAC) - Association of International Certified Professional Accountants
PRO TIP
If you happen to have plenty of certificates, select the ones that are most applicable and sought-after across the industry. Organize them by relevance to the role you're applying for.
Recommended reads:
Which one to use: a resume summary or a resume objective?
The regulatory affairs resume summary or objective serves as a good introduction to your experience for recruiters.
Have you ever wondered which one (the summary or objective) will be more appropriate for your regulatory affairs resume?
- If you are a less experienced professional, write a resume objective statement. The objective is about three sentences long and provides recruiters with information about your career goals, strengths, and achievements . It should basically denote how you see yourself in this particular role, and what is your relevant experience and/or know-how;
- If you happen to have plenty of relevant experience, select your most impressive achievements for your resume summary. The summary is no longer than five sentences and serves as a storytelling instrument - highlighting your greatest career wins . Don't forget to align your summary with the job requirements to ensure your resume stays relevant to the role.
Read on for more information and examples of resume summaries and objectives from real world professionals.
Resume summaries for a regulatory affairs job
- Accomplished regulatory affairs professional with over a decade of experience in leading the successful submission of multiple drug applications, instrumental in steering the pivotal phase III trials for a groundbreaking oncology therapy. Adept in FDA and EMA guidelines, committed to ensuring stringent compliance within a fast-paced biotech environment.
- Dedicated pharmacist transitioning into regulatory affairs, leveraging an 8-year background in community and hospital pharmacy settings, extensive knowledge of compounding and distribution regulations, and a keen interest in applying these skills to the regulatory domain to facilitate the strategic development of compliant healthcare products.
- Seasoned engineer with 12 years in aerospace project management, seeking to bring a robust analytical skillset and a history of cross-functional team leadership to the regulatory sector. Proven track record in achieving ISO certifications and implementing rigorous quality control systems that exceed industry standards.
- Eager recent graduate with a Master's degree in Biochemistry and internships at top-tier research laboratories, ready to contribute a fresh perspective and scientific acumen to the field of regulatory affairs. Demonstrated aptitude in data analysis and a passion for regulatory science, aiming to ensure public access to safe and effective medical innovations.
- With a fervent interest in the intersection of law and science, I am determined to leverage my legal studies background and in-depth understanding of healthcare legislation to facilitate the successful navigation of regulatory pathways. Proactive and analytical, I aim to commence my career in regulatory affairs by significantly contributing to the advancement of public health initiatives.
- As a dynamic professional with a strong foundation in environmental policy and a recent certification in regulatory affairs, I aspire to integrate my skills in sustainability with the robust framework of pharmaceutical compliance. Enthusiastic about championing environmental health and safety regulations in a new capacity, I am set to embark on a dedicated path in regulatory affairs.
Optimize your resume summary and objective for ATS
Drop your resume here or choose a file.
PDF & DOCX only. Max 2MB file size.
Miscellaneous regulatory affairs resume sections for a more personalized approach
Your regulatory affairs resume can reflect even more upon your personality and best qualities - that is if you decide on including a couple of additional resume sections to support your application.
Some of the best-accepted industry-wide choices include the:
- Resume projects - getting into the outcomes of your most important work, so far;
- Languages on your resume - detailing your proficiency level;
- Special recognitions - dedicated to your most prominent industry awards;
- Hobbies and interests - defining how you spend your free time.
Key takeaways
- All aspects of your resume should be selected to support your bid for being the perfect candidate for the role;
- Be intentional about listing your skill set to be balanced with both technical and people capabilities, while aligning with the job;
- Include any experience items that are relevant to the role and ensure you feature the outcomes of your responsibilities;
- Use the summary or objective as a screenshot of your best experience highlights;
- Curate various resume sections to showcase personal, transferable skills.