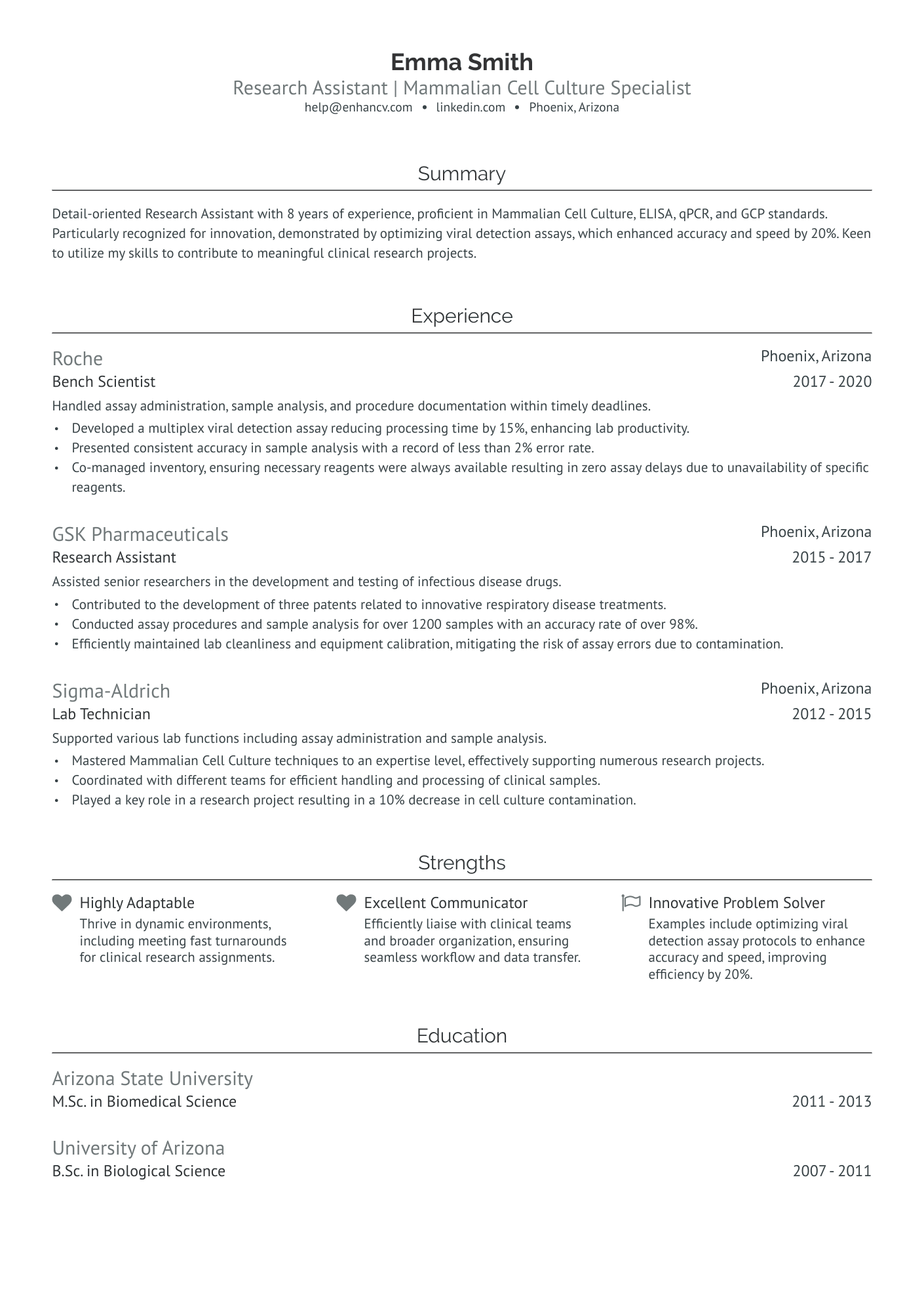
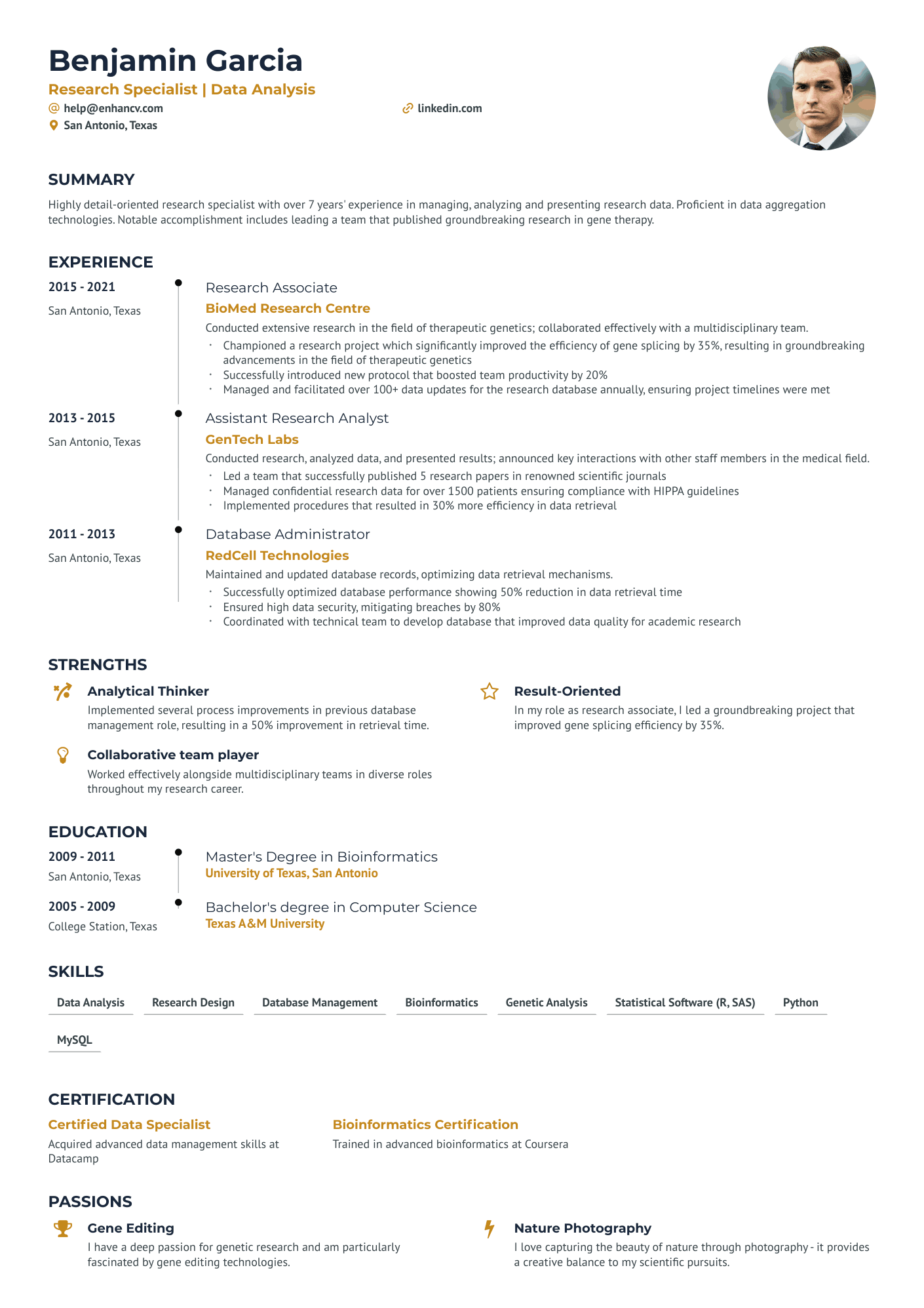
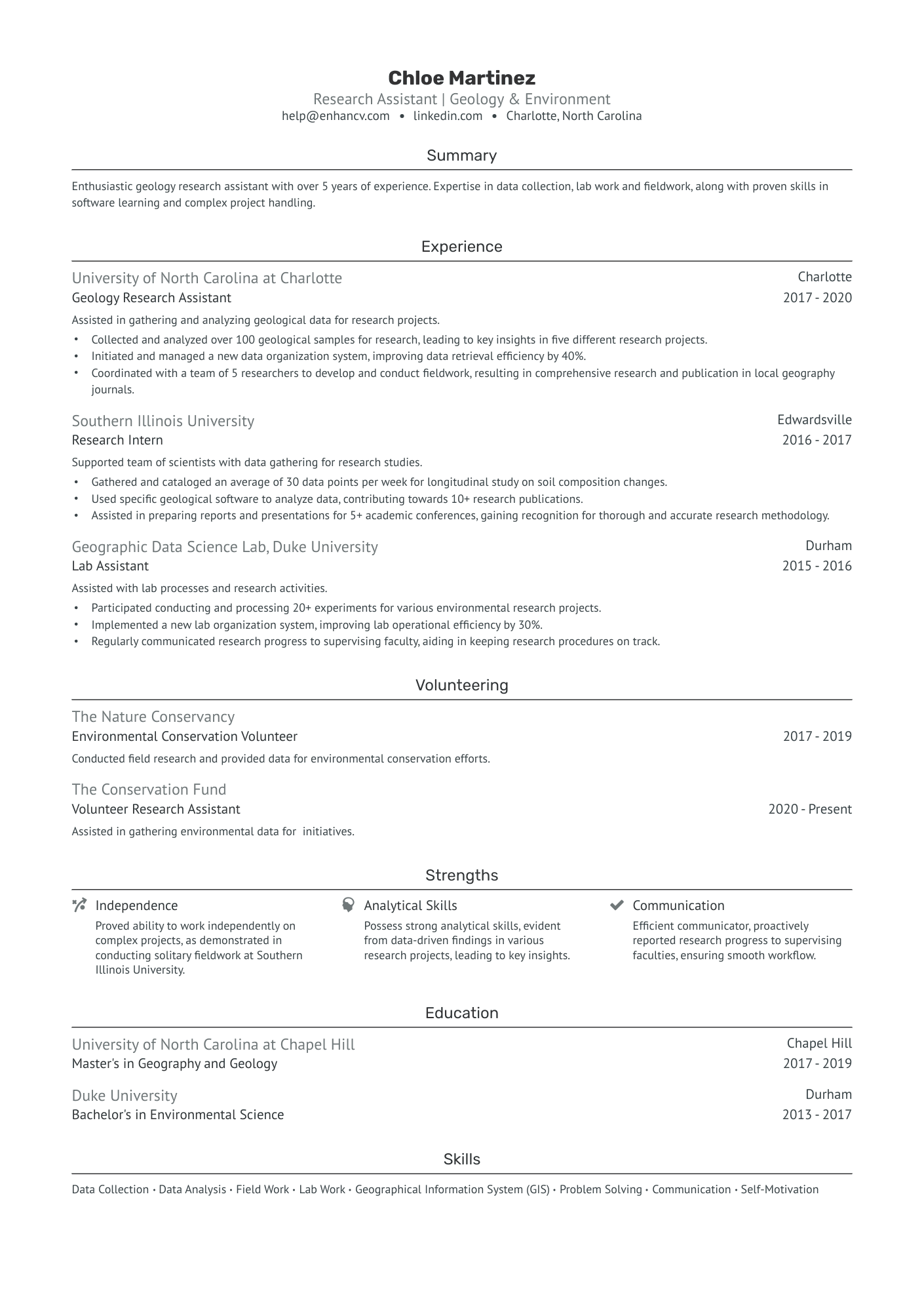
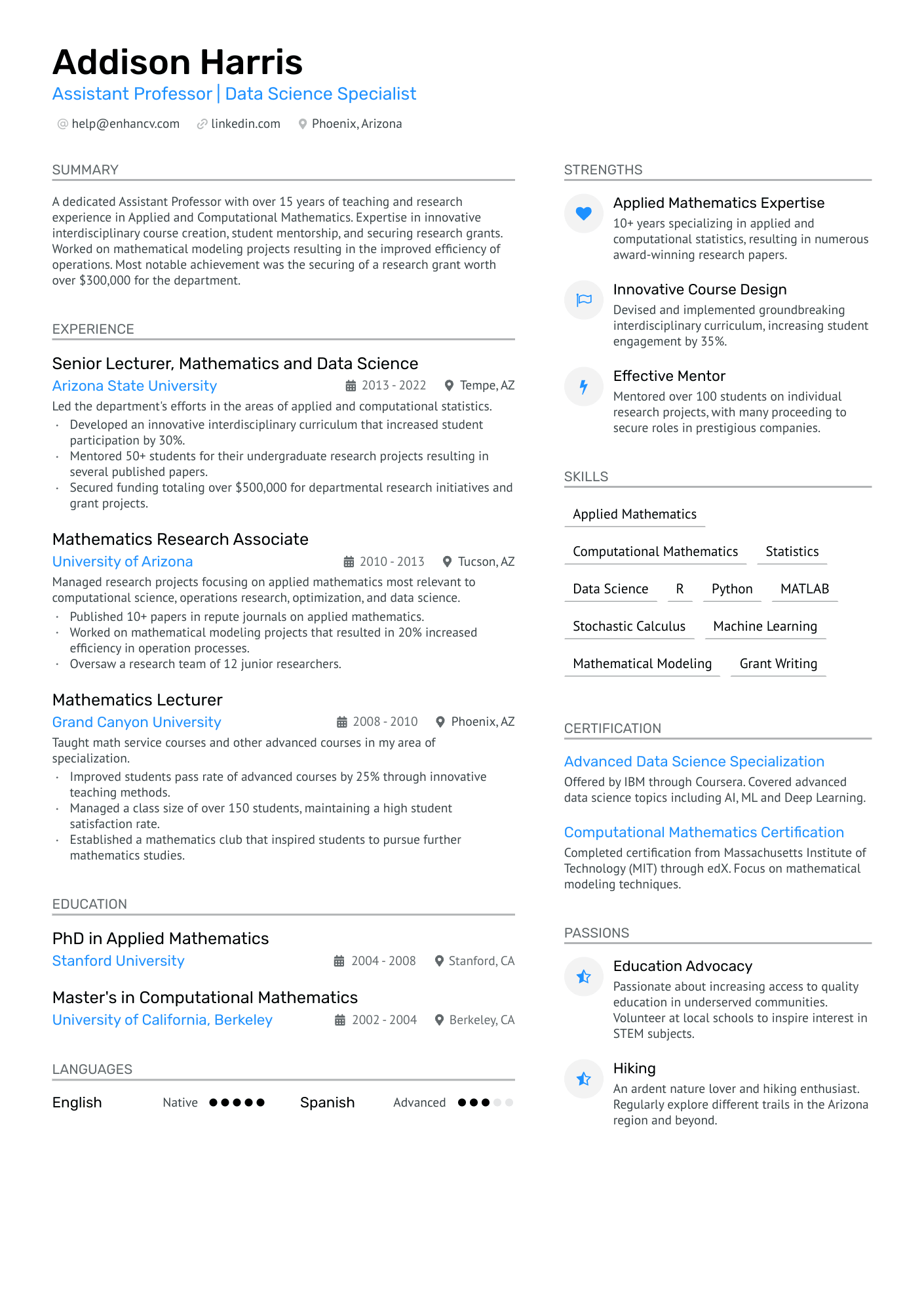
As a research assistant, articulating the breadth of your technical skills and academic research experience on a single-page resume can be a daunting challenge. Our guide offers tailored strategies and examples to help you effectively summarize your expertise and achievements, ensuring your resume stands out to potential employers.
- Sample industry-leading professional resumes for inspiration and research assistant resume-writing know-how.
- Focus recruiters' attention on what matters most - your unique experience, achievements, and skills.
- Write various resume sections to ensure you meet at least 95% of all job requirements.
- Balance your research assistant technical expertise with personality to stand out amongst candidates.
If the research assistant resume isn't the right one for you, take a look at other related guides we have:
The importance of format and layout in your research assistant resume
Consider you're an HR professional at company X, evaluating two research assistant candidate resumes. John Smith presents a simple, traditional, and easy-to-read resume. Edward Price, however, uses a non-conventional, often illegible format. Whose resume would you spend more time on to understand their experience? This scenario underscores the importance of your research assistant resume’s design. It should be simply formatted and clearly communicate why you are the ideal candidate for the role.
Achieve this balance by:
- Listing your experience, beginning with the most recent and relevant, in reverse chronological order;
- Ensuring your header contains essential information, such as contact details, a headline, and a portfolio link. Include a professional photo in the research assistant resume header if you have one;
- Including only the most important and relevant resume sections to showcase your expertise and stand out from other candidates;
- Editing your research assistant resume to be no longer than two pages if you have extensive relevant experience. Use your limited resume space judiciously.
Also, remember that your research assistant resume might initially be scanned by an Applicant Tracker System (ATS).
When it comes to ATS:
- Opt for simple and legible fonts like Raleway, Rubik, Lato, etc., making your experience easy for the ATS to scan;
- Use serif and sans-serif fonts, both of which are ATS-friendly;
- Avoid overused options like Arial and Times New Roman, which, while suitable, may lack personality.
Contrary to a common myth, our recent study shows that the ATS can effectively process both one-column and two-column resumes. Learn more about this in the ATS myths guide.
Finally, when submitting your research assistant resume, always export it as a PDF to ensure all information remains intact, making the document easier to print, read, and scan.
Customize your resume for the market – a Canadian format, for example, might vary in structure.
Upload & Check Your Resume
Drop your resume here or choose a file. PDF & DOCX only. Max 2MB file size.
PRO TIP
If you failed to obtain one of the certificates, as listed in the requirements, but decide to include it on your resume, make sure to include a note somewhere that you have the "relevant training, but are planning to re-take the exams". Support this statement with the actual date you're planning to be re-examined. Always be honest on your resume.
The six in-demand sections for your research assistant resume:
- Top one-third should be filled with a header, listing your contact details, and with a summary or objective, briefly highlighting your professional accolades
- Experience section, detailing how particular jobs have helped your professional growth
- Notable achievements that tie in your hard or soft skills with tangible outcomes
- Popular industry certificates to further highlight your technical knowledge or people capabilities
- Education to showcase your academic background in the field
What recruiters want to see on your resume:
- Proven experience with research methodologies and data analysis tools relevant to the field.
- Demonstrable experience in literature reviews, data collection, and reporting research findings.
- Proficiency in statistical software (e.g., SPSS, R, STATA) or scientific software (depending on the research field).
- Record of publications or contributions to academic journals (if applicable).
- Strong organizational skills, with the ability to manage multiple tasks and maintain meticulous attention to detail.
What to include in the experience section of your research assistant resume
The resume experience section is perhaps the most important element in your application as it needs to showcase how your current profile matches the job.
While it may take some time to perfect your research assistant experience section, here are five tips to keep in mind when writing yours:
- Assess the advert to make a list of key requirements and look back on how each of your past jobs answers those;
- Don't just showcase you know a particular skill, instead, you need proof in the form of tangible results (e.g. numbers, percent, etc.);
- It's perfectly fine to leave off experience items that don't bring anything extra to your skill set or application;
- Recruiters want to understand what the particular value is of working with you, so instead of solely featuring technologies, think about including at least one bullet that's focused on your soft skills;
- Take care with wording each bullet to demonstrate what you've achieved, using a particular skill, and an action verb.
The below research assistant resume examples can help guide you to curate your professional experience, following industry-leading tips and advice.
- Coordinated a team of 5 junior research assistants for a groundbreaking study on gene editing, which resulted in a 30% increase in lab productivity.
- Managed the laboratory’s budget, reducing costs by 15% through strategic procurement and efficient resource utilization while maintaining research integrity.
- Authored and co-authored 3 research papers published in high-impact scientific journals, increasing the recognition of the laboratory's contributions to the biotechnology field.
- Facilitated the successful completion of Phase III clinical trials for an innovative Alzheimer’s medication, which involved the coordination of 200+ patient assessments.
- Implemented a new electronic data capture system that enhanced data accuracy by 40% and expedited reporting timelines for regulatory submissions.
- Trained 10 new staff members on clinical protocols and compliance standards, increasing the team's efficiency and adherence to FDA regulations.
- Developed an algorithm for DNA sequence analysis that improved the mapping accuracy by 25% compared to existing methods.
- Participated in a collaborative project which combined computational and experimental approaches to investigate the protein structures of disease pathogens.
- Presented research findings at three international conferences, enhancing the lab's reputation in the computational biology community.
- Synthesized over 50 organic compounds which led to the identification of two promising candidates for new drug development projects.
- Maintained the laboratory’s organic chemistry equipment with 98% uptime, ensuring consistent research progress and safety compliance.
- Assisted in acquiring a research grant of $500,000 by presenting the project proposal to a panel of experts.
- Conducted field research which analyzed the impact of industrial pollutants on local ecosystems, influencing the community's approach to environmental policy making.
- Collected and analyzed over 1,000 soil and water samples, establishing a significant set of data for longitudinal environmental impact studies.
- Provided research support that contributed to the publication of a comprehensive report on regional environmental sustainability practices.
- Contributed to developing an AI-driven predictive model for patient outcomes which improved prognostic accuracy by 35%.
- Processed and analyzed large datasets using machine learning techniques to uncover trends in patient health data, aiding in personalized treatment planning.
- Collaborated with a cross-functional team to integrate AI tools into the broader healthcare data system, enhancing data accessibility for clinical research.
- Assisted in neuroimaging studies that mapped brain activity related to cognitive processes, contributing to five published papers on the topic.
- Managed the calibration and operation of fMRI equipment, ensuring optimal performance and reliability during high-stake experiments.
- Initiated a data-sharing program with other research institutions, enhancing the group's capacity for large-scale meta-analyses.
- Supported the development of new photovoltaic materials, which contributed to a 10% increase in solar cell efficiency in preliminary tests.
- Conducted over 200 experiments to test the durability and performance of novel materials under extreme conditions, providing critical data for further research stages.
- Assisted in drafting a patent application for a new composite material, which is currently undergoing the review process by the USPTO.
Quantifying impact on your resume
- Include the number of research projects completed to demonstrate productivity and experience.
- List the amount of data analyzed or samples processed to highlight analytical skills and attention to detail.
- Mention the percentage of efficiency improvement in processes or operations to show problem-solving abilities.
- Specify the size of research teams led or coordinated to indicate leadership and teamwork capabilities.
- Quantify the number of publications or presentations to showcase communication skills and expertise.
- Indicate the amount of grant money secured to display financial acumen and the ability to procure resources.
- State the number of experiments or tests designed to reflect creativity and innovation in research methods.
- Provide the size of the dataset used in analyses to convey experience with large-scale data and project magnitude.
Action verbs for your research assistant resume
Making the most of your little to none professional experience
If you're hesitant to apply for your dream job due to limited professional experience, remember that recruiters also value the unique contributions you can offer.
Next time you doubt applying, consider this step-by-step approach for your resume's experience section:
- Rather than the standard reverse chronological order, opt for a functional-based format. This shifts the focus from your work history to your achievements and strengths;
- Include relevant internships, volunteer work, or other non-standard experiences in your research assistant resume's experience section;
- Utilize your education, qualifications, and certifications to bridge gaps in your research assistant resume experience;
- Emphasize your interpersonal skills and transferable skills from various industries. Often, recruiters seek a personality match, giving you an advantage over other candidates.
Recommended reads:
PRO TIP
Highlight any significant extracurricular activities that demonstrate valuable skills or leadership.
Defining your unique research assistant skill set with hard skills and soft skills
In any job advertisement, a blend of specific technologies and interpersonal communication skills is typically sought after. Hard skills represent your technical expertise and indicate your job performance capacity. Soft skills, on the other hand, demonstrate how well you would integrate within the company culture.
Incorporating a balanced mix of both skill types in your research assistant resume is crucial. Here's how you can do it:
- In your resume summary or objective, incorporate up to three hard and/or soft skills. Make sure to quantify these skills with relevant or impressive achievements; less
- The skills section should list your technical know-how.
- The strengths section is an ideal place to quantify your competencies by focusing on the achievements facilitated by these skills.
Top skills for your research assistant resume:
Statistical Analysis Software (e.g., SPSS, R)
Data Management Systems (e.g., SQL, Excel)
Qualitative Data Analysis Tools (e.g., NVivo, Atlas.ti)
Survey Design Software (e.g., Qualtrics, SurveyMonkey)
Reference Management Software (e.g., EndNote, Zotero)
Laboratory Equipment Operation (specific to field)
Programming Languages (e.g., Python, MATLAB)
Data Visualization Tools (e.g., Tableau, Power BI)
Research Methodology Knowledge
Technical Writing
Critical Thinking
Attention to Detail
Communication Skills
Problem-Solving
Time Management
Team Collaboration
Adaptability
Organizational Skills
Interpersonal Skills
Creativity
PRO TIP
Always remember that your research assistant certifications can be quantified across different resume sections, like your experience, summary, or objective. For example, you could include concise details within the expertise bullets of how the specific certificate has improved your on-the-job performance.
What are the best certificates to add to your research assistant resume + how to curate your education section
The education and certification resume sections are the underdogs of your research assistant resume.
They showcase to recruiters that you've invested plenty of time to gain valuable and specific know-how, vital for growth.
As far as the resume education section is concerned:
- Detail only advanced education, specifying the institution and timeframe.
- Indicate your forthcoming graduation date if you're in the midst of your studies.
- Consider omitting degrees that don't align with the job's requirements.
- Offer a description of your academic journey if it underscores your notable achievements.
When curating your degrees and certificates on your research assistant resume:
- Select only accreditation that matters to the role
- Niche knowledge that could help you stand out as a candidate (as is within the past few years), should be listed towards the top of your resume
- Include any pertinent data for credibility (e.g. institute name, graduation dates, etc.)
- Irrelevant degrees and certifications shouldn't make it on your resume. Those include your high school diploma and any specializations that have nothing to do with the technical or soft skills that are required for the job
As a final note, if you feel tempted to exclude your education or certification from your resume, don't.
These two sections could help you have a better competitive edge over other candidates - hinting that your professional journey in the industry may be for a longer period of time.
Recruiters find all of these research assistant credentials impressive:
The top 5 certifications for your research assistant resume:
- Certified Clinical Research Professional (CCRP) - Society of Clinical Research Associates (SoCRA)
- Certified Research Administrator (CRA) - Research Administrators Certification Council (RACC)
- Certified IRB Professional (CIP) - Public Responsibility in Medicine and Research (PRIM&R)
- Project Management Professional (PMP) - Project Management Institute (PMI)
- Certified Data Management Professional (CDMP) - Data Management International (DAMA)
PRO TIP
The more trusted the organization you've attained your certificate (or degree) from, the more credible your skill set would be.
Recommended reads:
Practical guide to your research assistant resume summary or objective
First off, should you include a summary or objective on your research assistant resume?
We definitely recommend you choose the:
- Resume summary to match job requirements with most noteworthy accomplishments.
- Resume objective as a snapshot of career dreams
Both the resume summary and objective should set expectations for recruiters as to what your career highlights are.
These introductory paragraphs (that are no more than five sentences long) should help you answer why you're the best candidate for the job.
Industry-wide best practices pinpoint that the research assistant resume summaries and objectives follow the structures of these samples:
Resume summaries for a research assistant job
- With 5 years of dedicated experience in analytical chemistry research, I have honed a robust skill set in advanced spectroscopy and chromatography techniques, leading to my role in the groundbreaking development of a novel synthetic pathway for pharmaceutical compounds at ChemiCore Laboratories.
- Transitioning from a successful 7-year career as a software engineer, I have developed a unique perspective and strong computational skills, now eagerly pivoting towards biological data analysis to contribute to innovative cancer research initiatives at GlobalGen Research Center.
- Diligent and meticulous, I bring over 6 years of experience in environmental science to tackle the pressing challenges of climate change. My proficiency in statistical analysis and my lead authorship of a high-impact study on coral reef ecosystems at Oceanix Institute underscore my scientific rigor.
- After a decade as a foreign language educator, my adept communication and organizational skills transfer seamlessly to the realm of sociological research, where I am fully committed to employing qualitative research methods to enrich understanding of cultural dynamics at SocioStudy Group.
- As a freshly graduated biologist with a fervent interest in marine conservation, I am eager to apply my academic knowledge, including my thesis on sustainable fisheries, to support hands-on research and contribute towards preserving vital aquatic ecosystems at BlueWave Marine Research.
- Eager to leverage my recent Master's in Public Health, I am poised to dive into epidemiological research, bringing my strong foundation in biostatistics and my graduate project analyzing healthcare disparities, to aid in developing strategies for disease prevention and health promotion at HealthScope Institute.
Optimize your resume summary and objective for ATS
Drop your resume here or choose a file.
PDF & DOCX only. Max 2MB file size.
Miscellaneous research assistant resume sections for a more personalized approach
Your research assistant resume can reflect even more upon your personality and best qualities - that is if you decide on including a couple of additional resume sections to support your application.
Some of the best-accepted industry-wide choices include the:
- Resume projects - getting into the outcomes of your most important work, so far;
- Languages on your resume - detailing your proficiency level;
- Special recognitions - dedicated to your most prominent industry awards;
- Hobbies and interests - defining how you spend your free time.
Key takeaways
- The logic of your resume presentation should follow your career highlights and alignment with the role;
- Curate information within different sections (e.g. summary, experience, etc.) that helps highlight your strengths;
- Exclude from your resume irrelevant experience items - that way you'd ensure it stays no longer than two pages and is easy to read;
- Dedicate space within the summary, experience, and/or achievements to highlight precisely why you're the best candidate for the role via your previous success;
- Both your technical and people capabilities should also play a crucial role in building up your research assistant application. Prove your skill set in various resume sections.
Research Assistant resume examples
By Experience
Entry-Level Research Assistant
Junior Research Assistant
Experienced Research Assistant
Lead Research Assistant
Senior Research Assistant
Principal Research Assistant
By Role